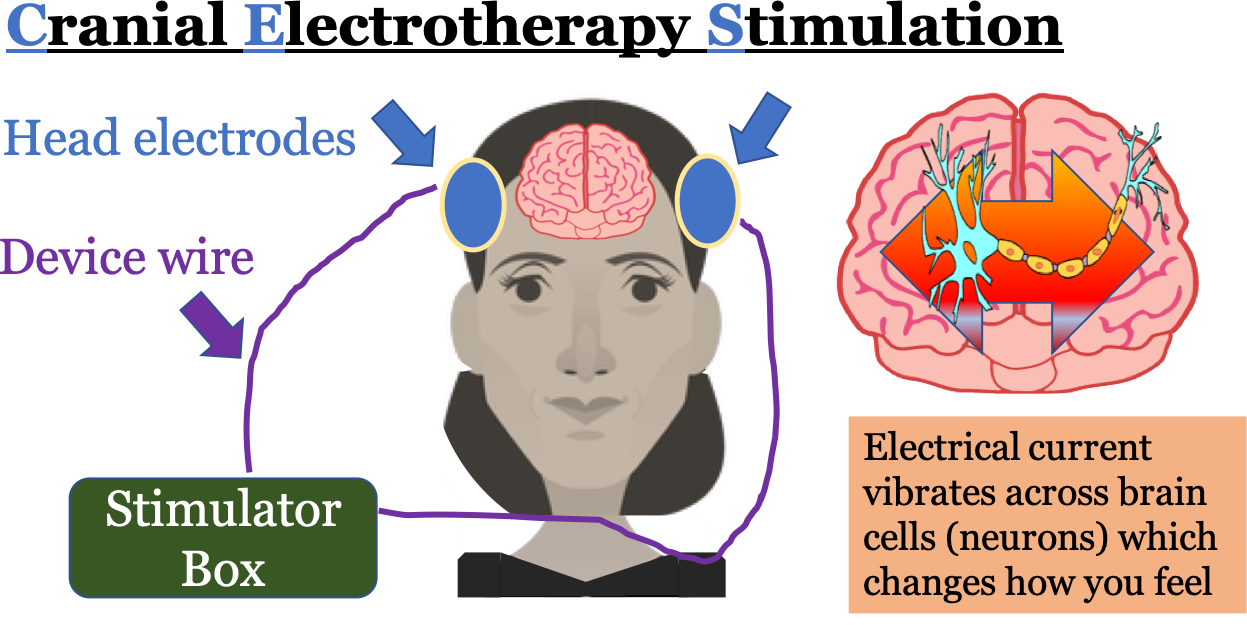
The basics of Cranial Electrotherapy Stimulation
CES is a class of non-invasive, transcranial pulsed-current stimulation that applies a particular amplitude, frequency, and waveform to patients. CES devices have two parts: a box to control settings, and a set of electrodes. Electrode placement is variable, but certain electrode arrangements have been cited by FDA-cleared devices, which can be viewed on the FDA website. Amplitude and frequency are also variable, with most CES devices allowing up to 5 mA and 0.5-500 Hz, respectively. Waveform can be square or sinusoidal. There is still debate on the efficacy of CES on its intended treatment targets of insomnia, anxiety, and depression. Although CES has been shown to affect neurotransmitter and neurohormone concentrations, while changing oscillatory band power in EEG recordings.2 Mental Health America (MHA) suggests clinical examination of CES in ADHD, anxiety, depression, addiction, pain, and insomnia. Additionally, CES hasn’t shown many side-effects in literature; vertigo, irritation, and headache are three which are noted.
Where Can I Get CES?
In the United States, the FDA has listed CES technology as a class III medical device. As such, a licensed medical practitioner is required for CES application or distribution. As of 2014, The FDA has announced a plan to reclassify CES devices as class II, but no action has yet been made.1 tDCS, TMS, tACS, ECT, CBS, and VNS all do not fall under the category of CES, and aren’t regulated as such.
The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
References
1. https://www.federalregister.gov/articles/2014/06/12/2014-13756/neurological-devices-withdrawal-of-proposed-effective-date-of-requirement-for-premarket-approval-for 2. Kirsch, D. L., & Nichols, F. (2013). Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatric Clinics of North America, 36(1), 169-176. 3. Mindes J, Dubin NJ, Altemus M (2014). Cranial Electrical Stimulation. In H Knotkova, D Rasche (Eds.) Textbook of Neuromodulation. New York: Springer, pp. 127-150.
